Vetter Pharma-Fertigung GmbH & Co. KG
About Vetter Pharma-Fertigung GmbH & Co. KG
Certifications
Categories

-
DE
-
2015On CPHI since
-
3Certificates
-
5000+Employees

Company types
Primary activities
Meet us at
CPHI Barcelona 2023
Fira Barcelona Gran Via, Spain
24-26 October 2023
Pharmapack Europe 2024
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
24 & 25 January 2024
Products from Vetter Pharma-Fertigung GmbH & Co. KG (4)
-
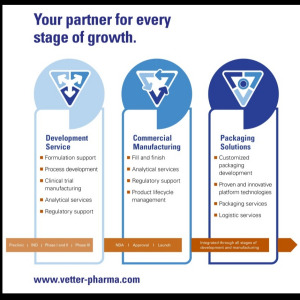
Product Vetter Development Service
Planning for success.
The period from preclinical through phase III is sometimes unpredictable for new molecules. With Vetter, you have a partner you can trust to support you throughout drug product development, clinical manufacturing and regulatory approval. We are ready to provide cli... -
Product Vetter Commercial Manufacturing
Quality across the supply chain
Commercial manufacturing of pharmaceutical and biotech drugs is a complex business today, requiring close integration of sophisticated resources and technologies to deliver top quality and solve supply chain challenges. Vetter’s decades of experience as... -
Product Vetter Packaging Solutions
Packaging matters.
At every stage of your product’s life cycle, there are many packaging questions to consider –from ensuring material compatibility to meeting the growing demand for patient-friendly systems.
Vetter can help you identify and develop the right packaging strategies f... -
Product Our Services
As a leading global CDMO, we offer comprehensive fill-and-finish services from clinical development to your product's market launch and beyond.
Vetter Pharma-Fertigung GmbH & Co. KG Resources (27)
-
News Globally-operating CDMO publishes annual sustainability report
Vetter again demonstrates industry leadership in sustainability initiatives -
Brochure Bringing your drug to the market
As experienced partner in injectable manufacturing, we support you every step of the way, guiding your product through development, regulatory approval, launch, market supply and life cycle management, accelerating your ability to bring new drugs to market -
News High demand: Vetter expands production capacities and services offered at its Austrian site
Pharma service provider further expands offerings for clinical development and manufacturing -
Brochure Comprehensive support for your injectables
We look at your project from every angle to help you find answers that work – answers that support your goals for quality, reliability, innovation, safety, and efficiency. When you partner with us, we integrate with your team to design and implement a personalized plan for success in a shifting global marketplace. -
News Gold Status for Vetter: Named Best Managed Company for the fourth time
CDMO honored as an exceptionally managed company -
Brochure Vetter Packaging Solutions
Delivering your product’s potential -
News Vetter strengthens worldwide commitment to sustainable business practices
CDMO joins United Nations Global Compact -
Brochure Vetter Commercial Manufacturing
Delivering results across the production chain -
News Vetter Performs Extraordinarily with Six Wins in the CDMO Leadership Awards
The CDMO Leadership Awards is an annual event now in its 12th year. In determining the award recipients, Life Science Leader teams up with (ISR) which conducted the research. For the 2023 award, more than 72 contract manufacturers were assessed by 23 performance metrics in ISR’s annual Contract Manufacturing Quality Benchmarking survey. Participants were recruited from pharma and biotech companies of all sizes and screened for decision-making influence related to working with contract manufacturing partners. Respondents only evaluate those companies with which they have worked on an outsourced project within the past 18 months. Through this level of qualification, survey responses are based on actual involvement with contract manufacturers and clear experiential data. -
Brochure Vetter Corporate Brochure
From clinical development to commercial production -
News Vetter joins United Nations Global Compact Network
Vetter (Ravensbury, Germany), the aseptic filling and packaging CDMO has signed up to the UN Global Compact Network. -
Brochure Vetter Development Service
Supporting your compounds success -
News Vetter’s Clinical Facility Demonstrates Success with Five Client Products Now on the Market
With the increasing drug development for novel therapeutics, a growing number of pharmaceutical and biotech companies are seeking clinical manufacturing partners to help meet the demand for complex fill-and-finish solutions. And, while the stages of early drug development are challenging, Vetter, a globally leading Contract Development and Manufacturing Organization (CDMO) is committed to addressing this challenge by offering comprehensive expert manufacturing services for clinical trials and supporting their customer products' path to commercialization. A testament to this commitment is evidenced by the success of its Skokie facility which has already contributed to bringing five new customer products to market since it began full operations in 2011, with another four expected to launch in the next months. -
Brochure Comprehensive support for your injectables
Build them together with Vetter.
Succeeding in today’s global pharmaceutical and biotech markets requires flexible thinking and precise manufacturing. No matter what phase of development your injectable product is in, we have solutions that can open new doors, shorten lead times, and solve complex challenges. At Vetter, we look at your project from every angle to help you find answers that work – answers that support your goals for quality, reliability, innovation, safety,and efficiency. When you partner with us, we integrate with your team to design and implement a personalized plan for success in a shifting global marketplace. -
News Strength in sustainability: Vetter wins gold in EcoVadis ranking
Vetter, a globally leading contract development and manufacturing organization (CDMO), achieved gold status in the renowned, independent EcoVadis sustainability ranking. With 72 points out of a maximum of 100, the pharmaceutical service provider significantly increased its score compared to the last evaluation and is now among the top five percent of all participating companies in the industry. -
Brochure Vetter at a glance
We’re a global expert in aseptic filling and packaging of injectable drug products – learn more about us. -
News New fully automated Vetter warehouse expands storage
Vetter, a globally leading contract development and manufacturing organization (CDMO), announced the commissioning of a new warehouse designed to help meet increasing customer market supply demands. -
Whitepaper Checklist Clinical Manufacturing
Get your product to the clinic with confidence
The shift from preclinical to clinical development is a critical step in the development of your drug product.
During this transition, you not only have to significantly upscale production of your product, but also make many strategic decisions that will lay important groundwork for your regulatory submission, commercial production of your product, and many other key components of your products life-cycle.
This eBook will help you build a plan that not only streamlines your products path to the clinic but also sets you up for long-term development and market success - starting with a proven 6-step process for filling your critical clinical batch.
-
News Vetter drives its sustainability campaign forward
Vetter, a globally leading contract development and manufacturing organization (CDMO), has in place a comprehensive sustainability strategy that has made it a pioneer in this energy-intensive industry. -
Video Customized packaging development
We offer a wide range of packaging services and injectable delivery systems that can be tailored to your product’s specific needs and optimize end-user convenience. -
News Vetter publishes first sustainability report
Pharmaceutical service provider integrates sustainability goals into its corporate strategy
-
Video V-CRT®️ - an integrated cleanroom concept
This innovative concept is based on the central idea of fully automated decontamination of the entire cleanroom, including RABS, using hydrogen peroxide (H2O2). V-CRT®️ is a two-barrier system that minimizes the risk of contamination and offers a number of advantages over existing concepts. -
News CPHI Trend Report - CDMO Opportunities in the Chinese Market
Opportunities for contract development and manufacturing organisations (CDMOs) in China continue to grow but many questions remain over what the future of the sector will look like in the world's second largest pharmaceutical market. -
Video Corporate Video
Comprehensive support for your injectable | Aseptic fill finish services at Vetter. -
Video CMO Leadership Awards 2022
Vetter wins the 2022 CMO Leadership Awards | Statement Sr. Vice President Carsten Press -
Video Oskar Gold - Market Developments
Market developments and multifaced impacts (Interview with Oskar Gold) -
Podcast Podcast: Drug Development-The Next Frontier for CDMOs?
The increasing demand for the manufacturing of highly complex biologics has driven many biotech and pharma companies to seek alliances with CDMOs. A qualified CDMO can help to understand business priorities, develop strategies to save time and costs, and provide regulatory guidance. This, in turn, means that CDMOs must have the right approach in place to fulfil high-quality requirements and have the right manufacturing technologies and capabilities in place. The complexity of CDMOs’ work will increase with new markets, demographic changes, and new demands in the clinical production of pharmaceuticals. This podcast was originally aired as part of the CPHI Festival of Pharma.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





















-file129092.png)